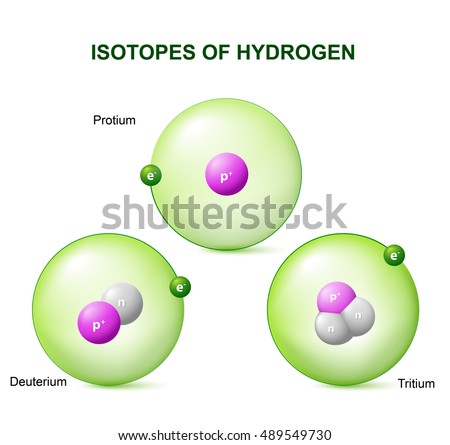
Isotopes
What is an isotope?
The isotopes are atoms whose their nucleus have the same number of protons but different number of neutrons, this means their atomic number is the same but their atomic mass is different. Not all the atoms of the same element are identical and each of these varies in a different isotopes.
Discoverement:
The existence of the isotopes was discovered by F. Soddy in 1911. Soddy noticed the similarities between their chemical properties
Most of the natural elements are formed by several isotopes that can only be separated by physical procedures.
Most of the natural elements are formed by several isotopes that can only be separated by physical procedures.
Classification:
We can classify isotopes as stables, that can exist around 3 billion years and unstables or radioactives, which emit radiation. These are used in obtaining electrical energy through nuclear fission reactions, in measuring apparatus, etc.
The atomic mass of an element in the periodic table is the average of the isotopes that has been discovered in a natural way.
Types:
There are two types of isotopes: the light isotopes usually have less neutrons that protons if they are not the same; and the heavy isotopes, they usually have more neutrons than protons.
You can find isotopes with a only a few or a lot of neutrons, this atoms can only exist for a while, but they are unstable. These are radioactive: their nucleus change or they desintegrate emitting radiations.
How are they representated?
There are two ways of representing them: according to scientific notation and according to symbolic notation.
Scientific Notation: The isotopes are identified by the name of the element followed by the number of protons and neutrons of the isotope.
Ex: U-234, U-235 and U-238
Symbolic Notation: The number of protons and neutrons is denoted as the superscript prefix of the chemical symbol.
Ex: 234U, 235U and 238U
Hope you have learn with our blog of isotopes.
Juan Sebastian Olarte- Gabriel Rios 8-40
You can find isotopes with a only a few or a lot of neutrons, this atoms can only exist for a while, but they are unstable. These are radioactive: their nucleus change or they desintegrate emitting radiations.
How are they representated?
There are two ways of representing them: according to scientific notation and according to symbolic notation.
Scientific Notation: The isotopes are identified by the name of the element followed by the number of protons and neutrons of the isotope.
Ex: U-234, U-235 and U-238
Symbolic Notation: The number of protons and neutrons is denoted as the superscript prefix of the chemical symbol.
Ex: 234U, 235U and 238U
Hope you have learn with our blog of isotopes.
Juan Sebastian Olarte- Gabriel Rios 8-40